
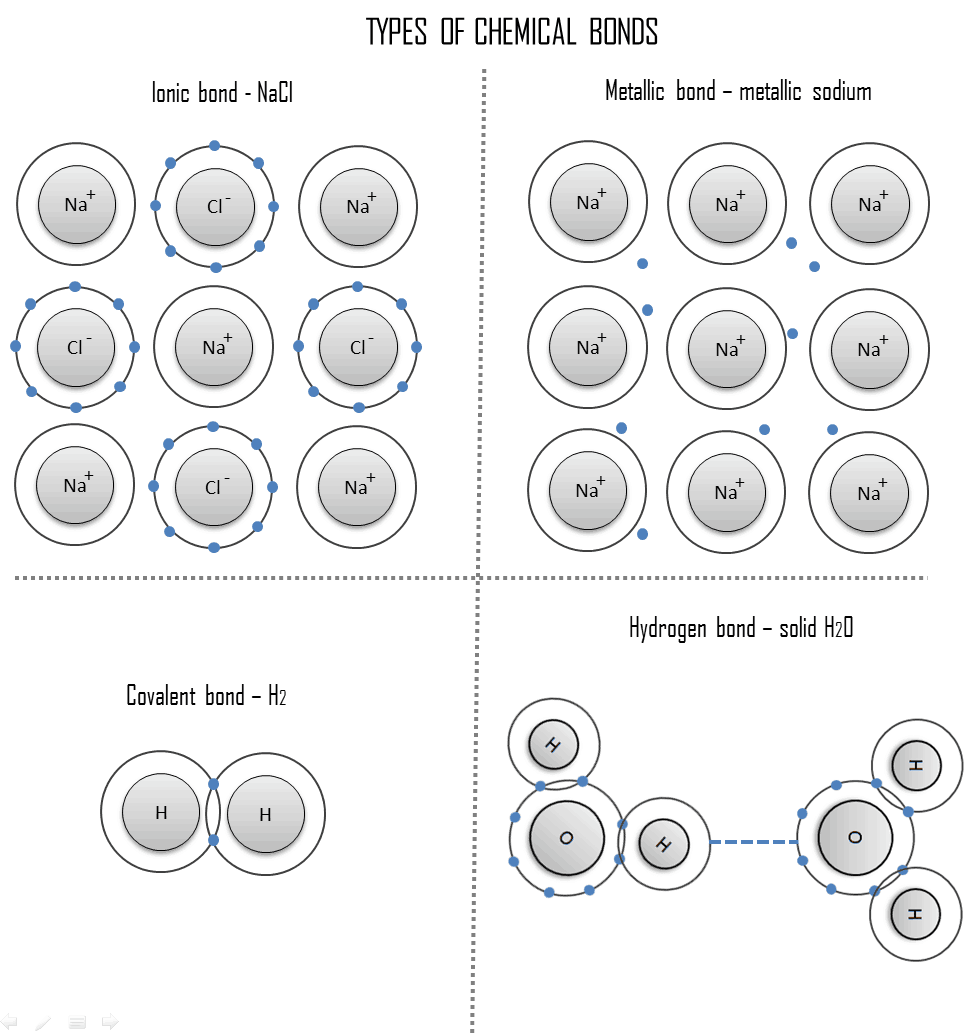
Low-melting solids and compounds that are liquids at room temperature are usually covalent. The higher-melting substance contains stronger bonds between its particles the lower-melting substance has comparatively weaker interparticle bonds. The melting and boiling points of substances of similar molecular weight are a measure of the relative strength of their intermolecular, interatomic, or interionic bonds. The bonds between the atoms, ions, or molecules in a substance range from very strong to comparatively weak. It does not break the bonds within molecules, only those between molecules. The added energy counteracts the forces that held the water molecules in the rigid ice structure. For example, when 6.02 kJ of energy are added to 1 mol (18 g) of ice at 0☌, there is no change in temperature, only a change in state from solid to liquid. Instead the energy that is added or subtracted breaks or forms bonds. When the potential energy of a sample changes, the temperature does not change. Of a sample changes, its temperature changes. These attractive forces also represent a part of the potential or stored energy of a sample.
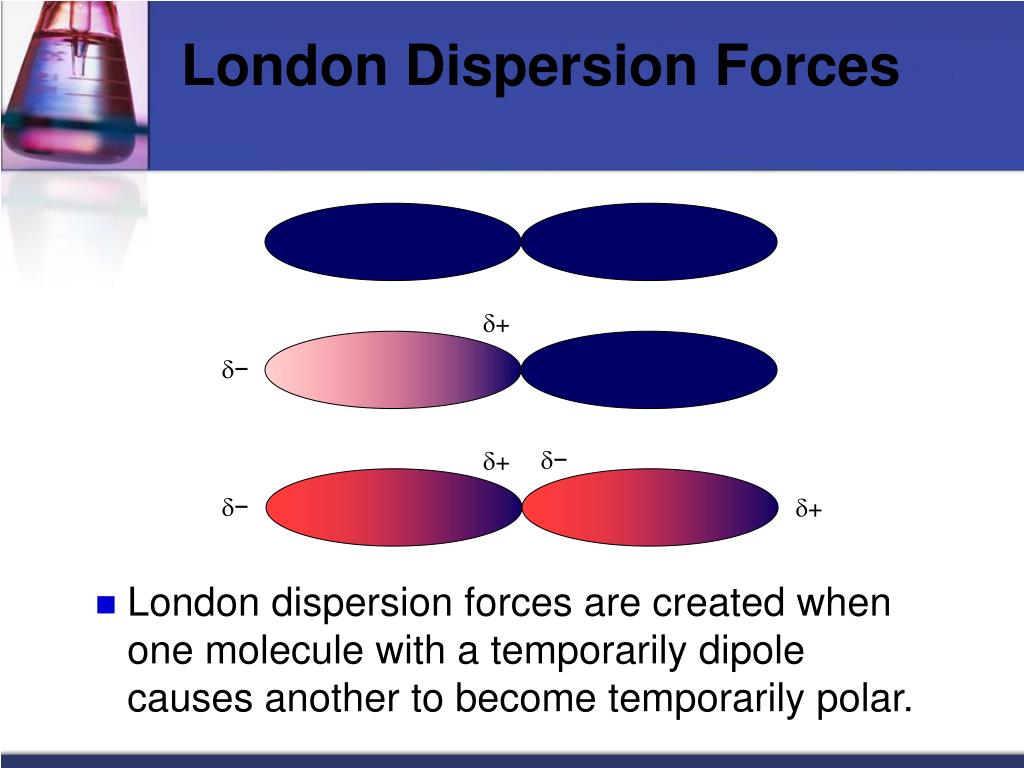
The magnitude of the attraction of one particle for another is important in determining whether the substance containing those particles is a solid, a liquid, or a gas under normal conditions (20☌, 1 atm).


 0 kommentar(er)
0 kommentar(er)
